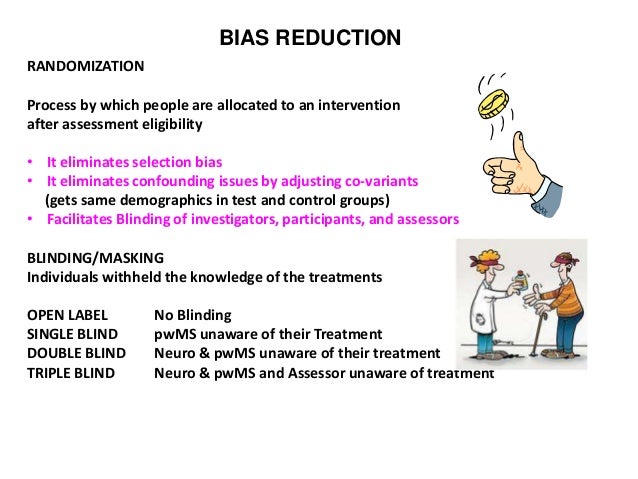
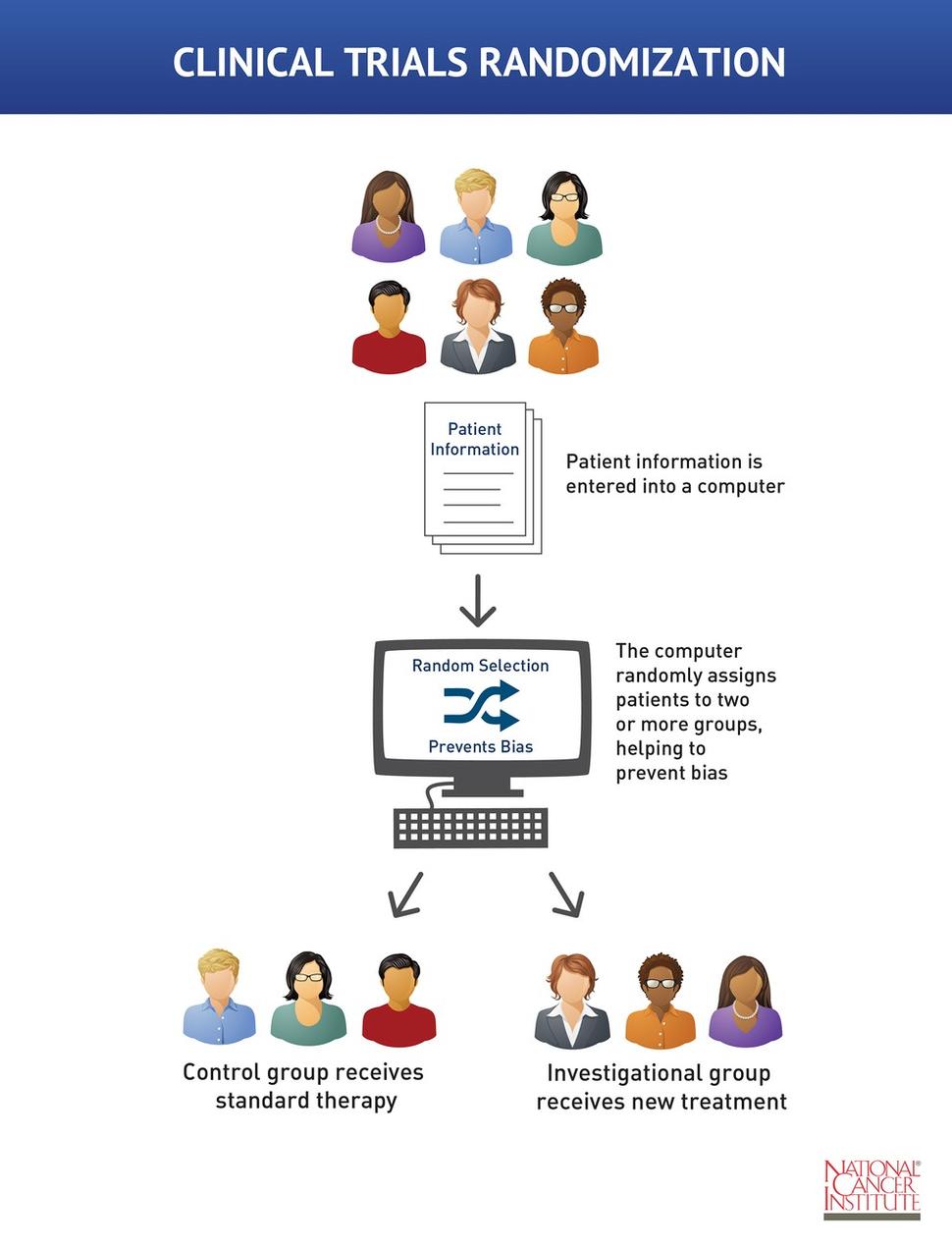
Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014-16: cross sectional analysis | The BMJ
![PDF] The Clinical Viewpoint: Definitions, Limitations of RECIST, Practical Considerations of Measurement | Semantic Scholar PDF] The Clinical Viewpoint: Definitions, Limitations of RECIST, Practical Considerations of Measurement | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0d341492d445073e64d1ccb6985e4ad763a3d1f7/2-Table1-1.png)
PDF] The Clinical Viewpoint: Definitions, Limitations of RECIST, Practical Considerations of Measurement | Semantic Scholar

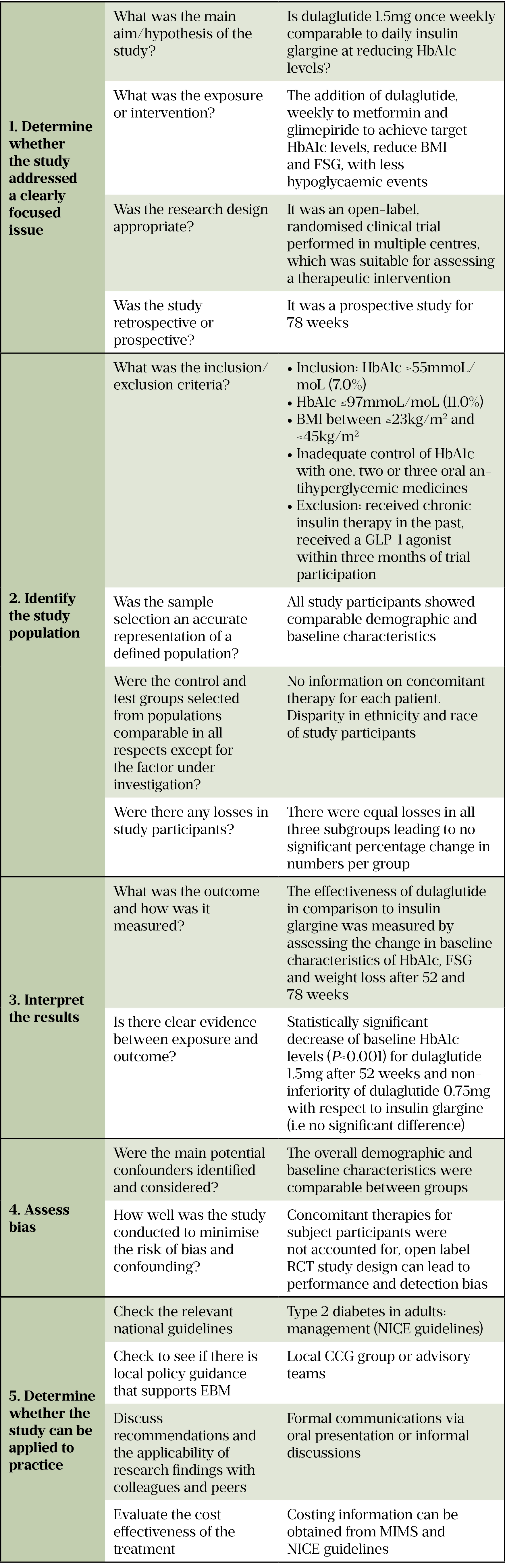
Hydroxychloroquine for COVID-19: What do the clinical trials tell us? - The Centre for Evidence-Based Medicine

Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014-16: cross sectional analysis | The BMJ

Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014-16: cross sectional analysis | The BMJ
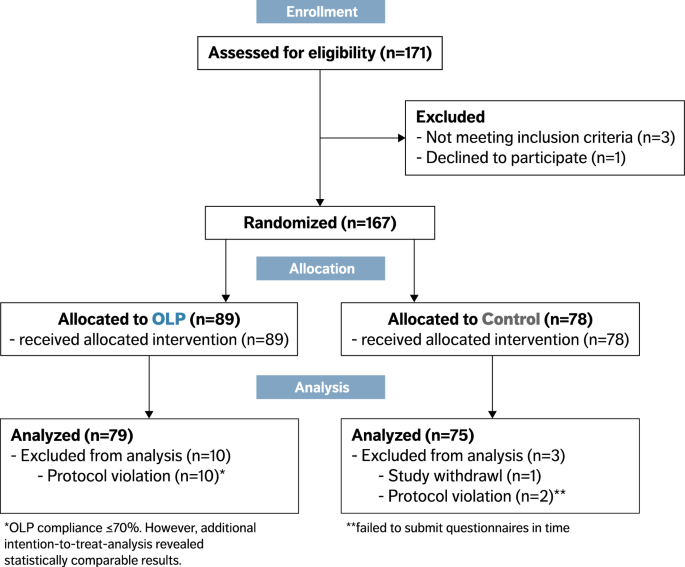
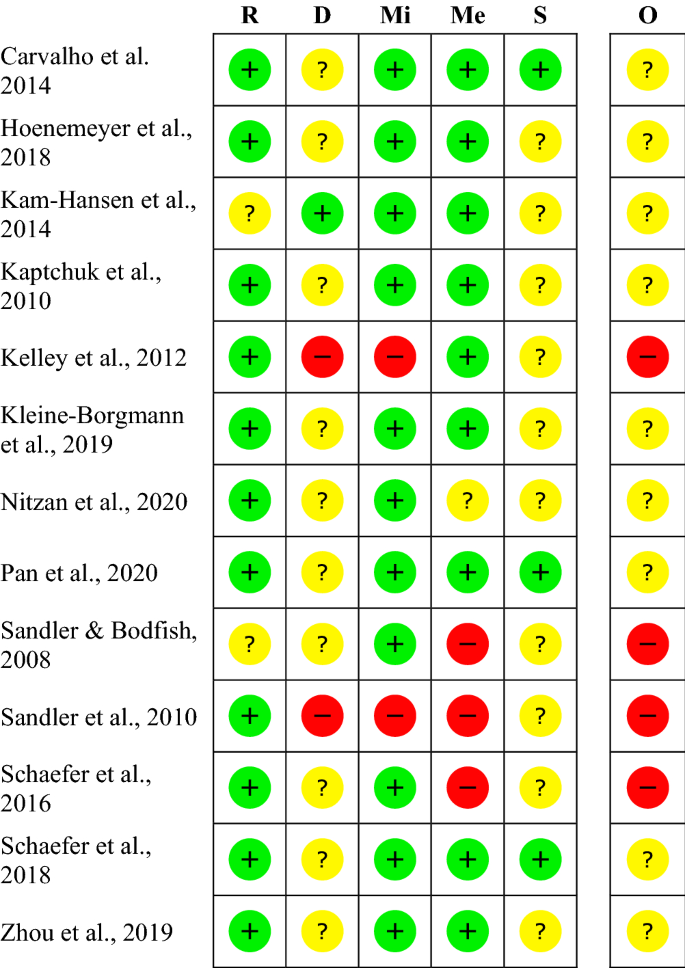
Effects of open-label placebos on test performance and psychological well-being in healthy medical students: a randomized controlled trial | Scientific Reports

Clinical trial design in chronic obstructive pulmonary disease: current perspectives and considerations with regard to blinding of tiotropium – topic of research paper in Clinical medicine. Download scholarly article PDF and read

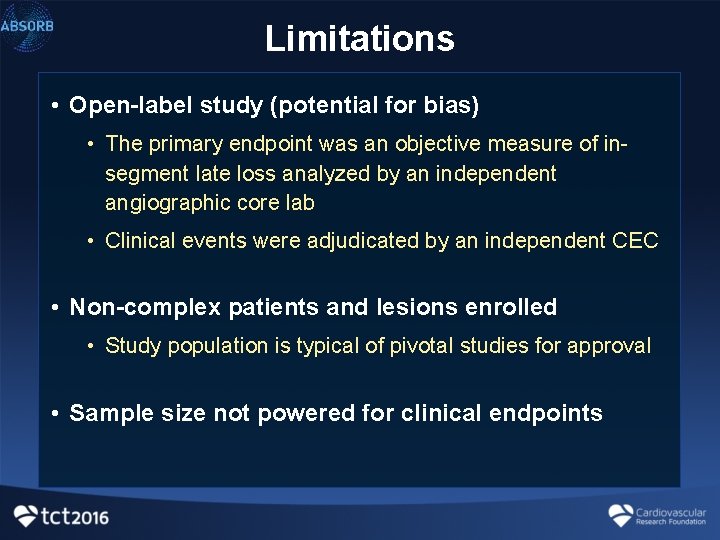
Statistical controversies in clinical research: limitations of open-label studies assessing antiangiogenic therapies with regard to evaluation of vascular adverse drug events—a meta-analysis - Annals of Oncology